Description
HSP70 and HSP90 are molecular chaperones expressed constitutively under normal conditions to maintain protein homeostasis and are induced upon environmental stress. Both HSP70 and HSP90 are able to interact with unfolded proteins to prevent irreversible aggregation and catalyze the refolding of their substrates in an ATP- and co-chaperone-dependent manner. HSP70 has a broad range of substrates including newly synthesized and denatured proteins, while HSP90 tends to have a more limited subset of substrates, most of which are signaling molecules. HSP70 and HSP90 often function collaboratively in a multi-chaperone system, which requires a minimal set of co-chaperones: HSP40, Hop, and p23 . The co-chaperones either regulate the intrinsic ATPase activity of the chaperones or recruit chaperones to specific substrates or subcellular compartments. When the ubiquitin ligase CHIP associates with the HSP70/HSP90 complex as a cofactor, the unfolded substrates are subjected to degradation by the proteasome. The biological functions of HSP70/HSP90 extend beyond their chaperone activity. They are essential for the maturation and inactivation of nuclear hormones and other signaling molecules. They also play a role in vesicle formation and protein trafficking.
Product
50mM Tris-Glycine (pH 7.4), 0.15M NaCl, 40% Glycerol, 0.01% Sodium azide and 0.05% BSA
Molecular Weight
~ 70 kDa
Swiss-Prot
P0DMV8
Purification & Purity
The antibody was purified by immunogen affinity chromatography.
Applications
WB (1/500 - 1/1000), IHC (1/50 - 1/100), IF/ICC (1/50 - 1/100)
Specificity:
Recognizes endogenous levels of HSP70 protein.
DATA
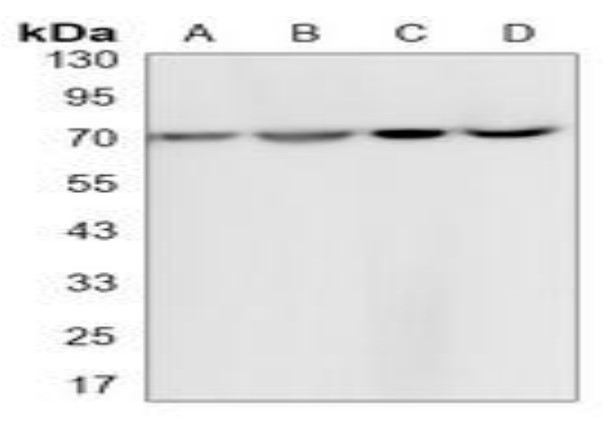
Western blot analysis of HSP70 expression in K562 (A), rat brain (B), C6 (C), NIH3T3 (D) whole cell lysates.
Storage & Stability
Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze-thaw cycles.
Note
For research use only, not for use in diagnostic procedure.



 <
< <
< <
<

 客服1
客服1  客服2
客服2